|
This article may be reprinted free of charge provided 1) that there is clear attribution to the Orthomolecular Medicine News Service, and 2) that both the OMNS free subscription link http://orthomolecular.org/subscribe.html and also the OMNS archive link http://orthomolecular.org/resources/omns/index.shtml are included. |
|||||
|
FOR IMMEDIATE RELEASE
The VICTAS Trial: Designed to Failby Michael Passwater(OMNS Mar 7, 2021) A recent clinical research article concludes, "Among critically ill patients with sepsis, treatment with vitamin C, thiamine, and hydrocortisone, compared with placebo, did not significantly increase ventilator- and vasopressor-free days within 30 days. However, the trial was terminated early for administrative reasons and may have been underpowered to detect a clinically important difference." [1] For some medical professionals, that study is proof that "HAT Therapy" (Hydrocortisone, Ascorbic acid, Thiamine), and vitamin C is not helpful in the treatment of sepsis. But such a conclusion is a dangerous over-generalization of the study's findings. Rather than focus on the early termination of the study, a more concerning aspect is its design. The treatment for the subjects included in the analysis was not required to begin quickly. The study treatments were given many hours (median 14.7) after subjects' sepsis symptoms worsened into cardiovascular or respiratory failure. The intravenous (IV) vitamin C dose was limited and fixed at 1.5g every 6 hours (86 mg/kg/day; 6g per day for a 70 kg subject), and the duration of treatment was limited to 4 days. The protocol did not require measurements of vitamin C, thiamine, or cortisol in study subjects before, during, or after treatment, and no measurements were reported in the article. Further, no measures of other co-nutrients were included. For instance, a low vitamin D level is an established biomarker of all-cause mortality in the ICU setting. [2] Low zinc, magnesium, and selenoprotein levels, as well as anemia, have also been associated with poor outcomes in critical care, including viral sepsis. [3-8] The article does not say whether the treatment and control groups were balanced at study entry with respect to vitamin C and other nutrient levels, nor whether adequate vitamin C was given to maintain plasma levels in the therapeutic range during the study. The "Limitations" section of the article acknowledges "...a higher dose or dosing based on plasma vitamin C concentrations might yield different results." In both the Test and Control arms of the study, the per protocol mortality before ICU discharge was 16.6% and 17.0% respectively (p=0.91), and at 180 days was 39.5% and 36.8% respectively (p=0.57). Neither standard treatment nor the delayed addition of low dose IVC for a short duration improved the poor survival of sepsis in this study. The overall conclusion that one can draw from this VICTAS trial is that vitamin C is safe, but that too little, too late, for too short of a duration is inadequate. 50 years ago, Dr. Frederick R. Klenner published a summary of his experience and prior publications. [9] He encouraged a daily IV dose of 350 - 700 mg vitamin C per kg of patient body weight (25,000 - 50,000 mg for a 70 kg / 154 lb subject), increasing the dose and frequency as necessary until the patient recovered: "It is a demonstrated principle that the production of histamine and other end products from deaminized cell proteins released by injury to cells are a cause of shock. The clinical value of ascorbic acid in combating shock is explained when we realize that the deaminizing enzymes from the damaged cells are inhibited by vitamin C. It has been shown by Chambers and Pollock [10] that mechanical damage to a cell results in pH changes which reverse the cell enzymes from constructive to destructive activity. The pH changes spread to other cells. This destructive activity releases histamine, a major shock producing substance. The presence of vitamin C inhibits this enzyme transition into the destructive phase. Clark and Rossiter [11] reported that conditions of shock and stress cause depletion of the ascorbic acid content of the plasma. As with the virus bodies, ascorbic acid also joins with the protein factor of these toxins effecting quick destruction. The answer to these emergencies is simple. Large amounts of ascorbic acid 350 mg to 700 mg per kg body weight given intravenously. In small patients, where veins are at a premium, ascorbic acid can easily be given intramuscularly in amounts up to two grams at one site. Several areas can be used with each dose given. Ice held to the gluteal muscles until red, almost eliminates the pain. We always reapply the ice for a few minutes after the injection. Ascorbic acid is also given, by mouth, as followup treatment. Every emergency room should be stocked with vitamin C ampoules of sufficient strength so that time will never be counted-as a factor in saving a life. The 4 gram, 20 cc ampoule and 10 gram 50 cc ampoule must be made available to the physician." The CITRIS-ALI study used 50 mg vitamin C per kg patient weight per treatment (200 mg/kg/day; 14g per day for a 70 kg subject) - more than double the dose used in the VICTAS Trial - yet less than one third of the upper range promoted by Dr. Klenner. Moreover, the CITRIS-ALI study showed a clear survival benefit (mortality was a secondary endpoint in that trial). [12] This dose of 200 mg/kg/day was also used by the earlier Phase I safety trial of IVC in sepsis. [13] Why, years later, did the VICTAS Trial choose to use less than half that dose? What would happen if a trial was done using efficacious doses -- those shown for over 70 years to help real people recover from critical illness? Doctors who utilize this protocol don't go back to treating patients without it. In the January 20, 2021 OMNS article "The Treatment of Infectious Disease Using Vitamin C and Other Nutrients" Margot DesBois nicely covers the early history of IVC use in serious illness. [14] In addition to Drs. Frederick Klenner, Claus Jungeblut, Robert Cathcart, and William McCormick, more recent clinical medicine pioneers including Drs. Hugh Riordan, Ron Hunningshake, AA Fowler, Paul Marik, and Joseph Varon can be added to the list. [15-21] Of note, the most successful published protocol for Covid-19 hospital treatment in the USA includes 3g IVC per dose along with a corticosteroid and thiamine every 6 hours, and the use of 25g IVC doses if rescue therapy is needed. And the treatments are not stopped at 96 hours. The idea that giving vitamin C beyond 96 hours might be dangerous has no scientific or clinical basis. See the full COVID-19 treatment plan, [22] and the Riordan Clinic IVC protocol. [23] As a reminder for those conducting and reviewing nutrient research, here are "rules" published by vitamin researcher Robert P. Heaney. [24]
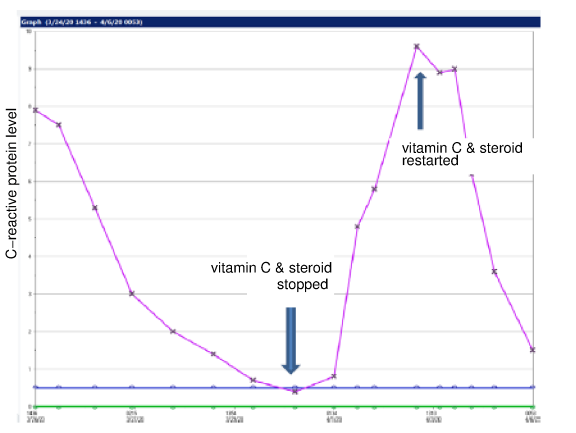
The VICTAS Trial [1] satisfied none of these 5 rules for conducting nutrient research. Recent research has shown the importance of vitamin C in sepsis and other acute life-threatening illnesses. Vitamin C has a multitude of essential for life effects within the human body, and due to its short half-life, is often the rate limiting factor in these biochemical processes. It is the primary extracellular antioxidant, and is important for scavenging damaging electron radicals. At very high levels it is involved in redox regulation, is a pro-oxidant, and can cause DNA and/or protein damage. This is useful in the treatment of cancer. It is an essential co-factor in the synthesis of catecholamines, vasopressin, steroids, neuropeptides and some neurotransmitters. It is also essential in the synthesis of collagen and elastin -- which are important molecules throughout the body, including in arteries and joints. Vitamin C is also important for epigenomic regulation of genes and is necessary for many cell types of the adaptive immune system. These biochemical functions are essential for improved immune cell function, endothelial cell function, hemodynamics (circulatory function), and wound healing. Stress, including cold temperatures, toxins, infections, and trauma greatly increase the cellular demand for vitamin C, and disrupt the body's ability to recycle oxidized vitamin C (dehydroascorbic acid or DHAA) back into the reduced form of vitamin C (ascorbic acid). Vitamin C has a short half life in the body (minutes to hours). In 2008, the prestigious journal Cell published the discovery that the red blood cells of humans (and other mammals unable to produce vitamin C) express a large number of GLUT1 transporters - more GLUT1 than on any other human cell type. [25] These GLUT1 transporters are apparently misnamed, as they might more properly be called DHAA1 transporters. The human RBC GLUT1 transporter is co-expressed with the protein stomatin which switches it into a DHAA transporter rather than a glucose transporter. [25] The result is 20-30 trillion red blood cells in healthy humans circulating through miles of blood vessels "soaking up" DHAA and - if adequate levels of the selenoprotein glutathione peroxidase are present in the red blood cells - reducing the DHAA back to AA and sending it back into the blood. A similar recycling system is present in the brain between astrocytes and tanycytes. [26] This supports the concept that keeping the blood, vasculature, and brain bathed in adequate ascorbic acid is important. Humans in acute distress from toxins, viruses, and bacteria have been successfully treated with high dose vitamin C injections for over 70 years. Recent studies have shown a synergistic benefit to endothelial cells when vitamin C and cortisol are injected into blood vessels simultaneously. Decades of experience have underscored the importance of early intervention, and increasing the dose and duration as needed to neutralize the acidosis and/or toxins. [27-53] Below is a graph courtesy of Dr. Paul E Marik of an ICU patient's c-reactive protein level (biomarker of inflammation) during 3g IVC and a corticosteroid co-administration every 6 hours for 96 hours, stopping the treatment, and then resuming the treatment. Continued vitamin C treatment until full recovery, tapering from IV to oral administration as the patient recovers, is important. It takes ongoing administration of vitamin C to achieve and maintain the tissue saturation levels needed to treat sepsis and septic shock.
Is 70 years of successful treatments to thousands of patients insufficient evidence? If more studies are needed, who will put the 350-700 mg/kg/day IVC dose to the test without the dangerous and artificial 96 hour limitation? Acknowledgements:I would like to acknowledge Benjamin Rakotoambinina, MD, PhD, professor of Physiology at the University of Antananarivo, Madagascar in collaboration with Laurent Hiffler, MD of the Cellular Nutrition Research Group for their critical review and feedback; and Drs. Robert G. Smith and Andrew Saul for their critical review and editorial support. (Michael E. Passwater, son of author and columnist Dr. Richard Passwater, is certified by the American Society for Clinical Pathology as a medical technologist, a specialist in immunohematology, and is a diplomate in laboratory management. He has worked in clinical laboratories for 28 years, and has previously written “Do the Math: "MATH+" Saves Lives†published by the Orthomolecular Medicine News Service http://orthomolecular.org/resources/omns/v16n55.shtml ). References:1. Sevransky JE, Rothman RE, Hager DN, et al. (2021) Effect of Vitamin C, Thiamine, and Hydrocortisone on Ventilator-and Vasopressor-Free Days in Patients With Sepsis: The VICTAS Randomized Clinical Trial. JAMA 325:742-751. https://jamanetwork.com/journals/jama/fullarticle/2776688 2. Moraes RB, Friedman G, Wawrzeniak IC, et al. (2015) Vitamin D deficiency is independently associated with mortality among critically ill patients. Clinics. 70:326-332. https://pubmed.ncbi.nlm.nih.gov/26039948 3. Alker W, Haase H. (2018) Zinc and Sepsis Nutrients 10:976. https://pubmed.ncbi.nlm.nih.gov/30060473 4. Noormandi A, Khalili H, Mohammadi M, et al. (2020) Effect of magnesium supplementation on lactate clearance in critically ill patients with severe sepsis: a randomized clinical trial. Eur J Clin Pharmacol 76:175-184. https://pubmed.ncbi.nlm.nih.gov/31814044 5. Velissaris D, Karamouzos V, Pierrakos C, et al. (2015) Hypomagnesemia in critically ill sepsis patients. J Clin Med Res 2015;7:911-918. https://pubmed.ncbi.nlm.nih.gov/26566403 6. Guerin C, Cousin C, Mignot F, et al. (1996) Serum and erythrocyte magnesium in critically ill patients. Intensive Care Med 22:724-727. https://pubmed.ncbi.nlm.nih.gov/8880238 7. Angstwurm MW, Engelmann L, Zimmermann T, et al. (2007) "Selenium in Intensive Care (SIC): results of a prospective randomized, placebo-controlled, multiple-center study in patients with severe systemic inflammatory response syndrome, sepsis, and septic shock." Crit Care Med. 35:118-26. https://pubmed.ncbi.nlm.nih.gov/17095947 8. Belsky JB, Wira CR, Jacob V, et al. (2018) A review of micronutrients in sepsis: the role of thiamine, l-carnitine, vitamin C, selenium and vitamin D. Nutr Res Rev. 31:281-290. https://pubmed.ncbi.nlm.nih.gov/29984680 9. Klenner FR. (1971) Observations On the Dose and Administration of Ascorbic Acid When Employed Beyond the Range of A Vitamin In Human Pathology. J Applied Nutrit. 23:61-87. https://seanet.com/~alexs/ascorbate/197x/klenner-fr-j_appl_nutr-1971-v23-n3&4-p61.htm 10. Chambers R, Pollock H. (1927) Micrurgical studies in cell physiology: IV. Colorimetric determination of the nuclear and cytoplasmic pH in th e starfish egg. J Gen. Physiol 10:739-755. https://pubmed.ncbi.nlm.nih.gov/19872358/ 11. Clark EJ, Rossiter RJ. (1944) Carbohydrate metabolism after burning. Q J Exp Physiol Cog Med Sci 32:279-300. https://doi.org/10.1113/expphysiol.1944.sp000890 12. Fowler AA, Truwit JD, Hite RD, et al. (2019) Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients With Sepsis and Severe Acute Respiratory Failure: The CITRIS-ALI Randomized Clinical Trial. JAMA 322:1261-1270. https://pubmed.ncbi.nlm.nih.gov/31573637 13. Fowler AA, Syed AA, Knowlson S, et al. (2014) "Phase I Safety trial of intravenous ascorbic acid in patients with severe sepsis." J Transl Med 12:32. https://pubmed.ncbi.nlm.nih.gov/24484547 14. DesBois M (2021) The Treatment of Infectious Disease Using Vitamin C and other Nutrients. Orthomolecular Medicine News Service. http://orthomolecular.org/resources/omns/v17n04.shtml 15. Klenner FR (1949) The Treatment of Poliomyelitis and other Virus Diseases with Vitamin C. South Med Surg. 111:209-214. https://pubmed.ncbi.nlm.nih.gov/18147027 https://vitamincfoundation.org/www.orthomed.com/polio.htm https://www.seanet.com/~alexs/ascorbate/194x/klenner-fr-southern_med_surg-1948-v110-n2-p36.htm 16. Jungeblut CW (1935) Inactivation of Poliomyelitis virus in vitro by crystalline vitamin C (ascorbic acid) J Exp Med. 62:517-521. https://pubmed.ncbi.nlm.nih.gov/19870431 17. Cathcart RF (1981) Vitamin C, titrating to bowel tolerance, anascorbemia, and acute induced scurvy. Med Hypotheses 7:1359-1376. https://pubmed.ncbi.nlm.nih.gov/7321921 18. McCormick WJ (1951) Vitamin C in the Prophylaxis and Therapy of Infectious Diseases. Arch Pediatr. 68:1-9. https://pubmed.ncbi.nlm.nih.gov/14800557 https://www.seanet.com/~alexs/ascorbate/195x/mccormick-wj-arch_pediatrics-1951-v68-n1-p1.htm 19. Hugh D Riordan HD, Hunninghake RB, Riordan NH, et al. (2003) Intravenous ascorbic acid: protocol for its application and use. P R Health Sci J, 22:287-90. https://pubmed.ncbi.nlm.nih.gov/14619456 20. Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J. (2017) Hydrocortisone, Vitamin C, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Before-After Study. Chest 151:1229-1238. https://pubmed.ncbi.nlm.nih.gov/27940189 21. Kory P, Meduri GU, Iglesias J, et al. (2021) Clinical and Scientific Rationale for the "MATH+" Hospital Treatment Protocol for COVID-19. J Intensive Care Med. 36:135-156. https://pubmed.ncbi.nlm.nih.gov/33317385 22. Front Line COVID-19 Critical Care Alliance (2021) EVMS COVID-19 Management Protocol: An overview of the MATH+ and I-MASK+ Protocols. http://www.flccc.net 23. Riordan H, Riordan, N, Casciari J (2021) The Riordan intravenous vitamin C (IVC) protocol for adjunctive cancer care: IVC as a chemotherapeutic and biologic response modifying agent. Riordan Clinic. https://riordanclinic.org/wp-content/uploads/2015/11/RiordanIVCprotocol_en.pdf 24. Heaney RP. (2014) Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr Rev 72:48-54. https://pubmed.ncbi.nlm.nih.gov/24330136 25. Montel-Hagen A, Kinet S, Manel N, et al. (2008) Erythrocyte Glut1 triggers dehydroascorbic acid uptake in mammals unable to synthesize vitamin C. Cell, 132:1039-1048. https://pubmed.ncbi.nlm.nih.gov/18358815 26. Nualart F, Mack L, GarcÃa A, et al. (2014) Vitamin C Transporters, Recycling and the Bystander Effect in the Nervous System: SVCT2 versus Gluts. J Stem Cell Res Ther 4:209. https://pubmed.ncbi.nlm.nih.gov/25110615 27. May JM, Harrison FE. (2013) Role of Vitamin C in the Function of the Vascular Endothelium. Antioxidants & Redox Signaling 19:2068-2083. https://pubmed.ncbi.nlm.nih.gov/23581713 28. Nabzdyk CS, Bittner EA. (2018) Vitamin C in the critically ill - indications and controversies. World J Crit Care Med 7:52-61. https://www.wjgnet.com/2220-3141/full/v7/i5/52.htm 29. Lee RE. (1961) Ascorbic Acid and the Peripheral Vascular System. Ann NY Acad Sci. 92:295-301. https://doi.org/10.1111/j.1749-6632.1961.tb46129.x 30. Lee RE, Holze EA. (1951) Nutritional factors in hemodynamics: dissociation of pressor response and hemorrhage resistance in avitaminosis C. Proc. Soc. Expt. Biol Med. 76:325-329. https://pubmed.ncbi.nlm.nih.gov/14827915 31. Barabutis N, Khangoora V, Marik PE, Catravas JD. (2017) Hydrocortisone and Ascorbic Acid Synergistically Prevent and Repair Lipopolysaccharide-Induced Pulmonary Endothelial Barrier Dysfunction. Chest 152:954-962. https://pubmed.ncbi.nlm.nih.gov/28739448 32. Parker WH, Rhea EM, Qu ZC. (2016) Intracellular ascorbate tightens the endothelial permeability barrier through Epac1 and the tubulin cytoskeleton. Am J Physiol Cell Physiol. 311:C652-C662. https://pubmed.ncbi.nlm.nih.gov/27605450 33. Gu W, Cheng A, Barnes H, et al. (2014) Vitamin C Deficiency Leading to Hemodynamically Significant Bleeding. JSM Clinical Case Reports 2:1046. https://www.jscimedcentral.com/CaseReports/casereports-2-1046.pdf 34. Zhao B, Fei J, Chen Y, et al. (2014) Vitamin C treatment attenuates hemorrhagic shock related multi-organ injuries through the induction of heme oxygenase-1. BMC Complementary and Alternative Medicine 14:442-454. https://pubmed.ncbi.nlm.nih.gov/25387896 35. Ladumer A, Schmitt CA, Schachner D, et al. (2012) Ascorbate stimulates endothelial nitric oxide synthase enzyme activity by rapid modulation of its phosphorylation status. Free Radic Biol Med. 52:2082-2090. https://pubmed.ncbi.nlm.nih.gov/22542797 36. Heller R, Munscher-Paulig F, Grabner R, Till V. (1999) L-Ascorbic Acid Potentiates Nitric Oxide Synthesis in Endothelial Cells. J Biol Chem 274:8254-8260. https://pubmed.ncbi.nlm.nih.gov/10075731 37. Dingchao H, Zhduan Q, Xiaodong F. (1994) The Protective Effects of High-Dose Ascorbic Acid on Myocardium against Reperfusion Injury During and After Cardiopulmonary Bypass. Thorac Cardiovasc Surg 42:276-278. https://pubmed.ncbi.nlm.nih.gov/7863489 38. Ichim TE, Minev B, Braciak T, et al. (2011) Intravenous ascorbic acid to prevent and treat cancer-associated sepsis? J Transl Med 9:25. https://pubmed.ncbi.nlm.nih.gov/21375761 39. Cisternas P, Silva-Alvarez C, Martinez F, et al. (2014) The oxidized form of vitamin C, dehydroascorbic acid, regulates neuronal energy metabolism. J Neurochem 129: 663-671. https://pubmed.ncbi.nlm.nih.gov/24460956 40. Wang Y, Lin H, Lin BW, et al. (2019) Effects of different ascorbic acid doses on the mortality of critically ill patients: a meta-analysis. Ann Intensive Care 9:58. https://pubmed.ncbi.nlm.nih.gov/31111241 41. Boretti A, Banik BK. (2020) Intravenous vitamin C for reduction of cytokines storm in acute respiratory distress syndrome. PharmaNutrition 12:100190. https://pubmed.ncbi.nlm.nih.gov/32322486 42. Iglesias J, Vassallo AV, Patel V et al. (2020) Outcomes of metabolic resuscitation using ascorbic acid, thiamine, and glucocorticoids in the early treatment of sepsis. Chest 158:164-173. https://pubmed.ncbi.nlm.nih.gov/32194058 43. de Melo AF, Homem-de-Mello M. (2020) High-dose intravenous vitamin C may help in cytokine storm in severe SARS-CoV-2 infection. Crit Care 24:500. https://pubmed.ncbi.nlm.nih.gov/32792018 44. Zhang J, Rao X, Li Y et al. (2021) Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Ann Intenisve Care 11:5. https://pubmed.ncbi.nlm.nih.gov/33420963 45. Lankadeva YR, Peiris RM, Okazaki N, et al. (2021) Reversal of the pathophysiological responses to Gram-negative sepsis by megadose Vitamin C. Crit Care Med 49:e179-e190. https://pubmed.ncbi.nlm.nih.gov/33239507 46. Patterson G, Isales CM, Fulzele S. (2021) Low level of vitamin C and dysregulation of vitamin C transporter might be involved in the severity of COVID-19 infection. Aging and Disease 12:14-26. https://pubmed.ncbi.nlm.nih.gov/33532123 47. Tomassa-Irriguible TM, Lielsa-Berrocal L. (2020) COVID-19: Up to 87% critically ill patients had low vitamin C values. Research Square, preprint. https://www.researchsquare.com/article/rs-89413/v1 48. Arvinte C, Singh M, Marik PE. Serum levels of vitamin C and vitamin D in a cohort of critically ill COVID-19 patients of a North American Community Hospital Intensive Care Unit in May 2020. A pilot study. Medicine in Drug Discovery 8:100064. https://pubmed.ncbi.nlm.nih.gov/32964205 49. Wagas Khan HM, Parikh N, Megala SM, Predeteanu GS. (2020) Unusual Recovery of a Critical COVID-19 Patient After Administration of Intravenous Vitamin C. Am J Case Rep 21: e925521. https://pubmed.ncbi.nlm.nih.gov/32709838 50. Marik PE. (2018) Hydrocortisone, Ascorbic Acid and Thiamine (HAT therapy) for the treatment of sepsis. Focus on ascorbic acid. Nutrients 10:1762. https://pubmed.ncbi.nlm.nih.gov/30441816 51. May JM, Qu ZC. (2011) Ascorbic acid prevents oxidant-induced increases in endothelial permeability. Biofactors 37:46-50. https://pubmed.ncbi.nlm.nih.gov/21328627 52. Utoguchi N, Ikeda K, Saeki K et al. (1995) Ascorbic acid stimulates barrier function of cultured endothelial cell monolayer. J Cell Physiol 163:393-399. https://pubmed.ncbi.nlm.nih.gov/7706381 53. Han M, Pendem S, Teh SL, et al. (2010) Ascorbate protects endothelial barrier function during septic insult: Role of protein phosphatase type 2A. Free Radic Biol Med 48:128-135. https://pubmed.ncbi.nlm.nih.gov/19840845 Click here to see a web copy of this news release: https://orthomolecular.acemlna.com/p_v.php?l=1&c=189&m=193&s=c7ae1002d2f579a22c16a1b89c854212 |
|||||
|
This news release was sent to sandra@positivehealth.com. If you no longer wish to receive news releases, please reply to this message with "Unsubscribe" in the subject line or simply click on the following link: unsubscribe . To update your profile settings click here . This article may be reprinted free of charge provided 1) that there is clear attribution to the Orthomolecular Medicine News Service, and 2) that both the OMNS free subscription link http://orthomolecular.org/subscribe.html and also the OMNS archive link http://orthomolecular.org/resources/omns/index.shtml are included.
|
|||||